27+ How To Determine Central Atom
Chemists use Lewis dot structures to represent the bonding schemes in molecules. Um So starting apart A we have a three P.
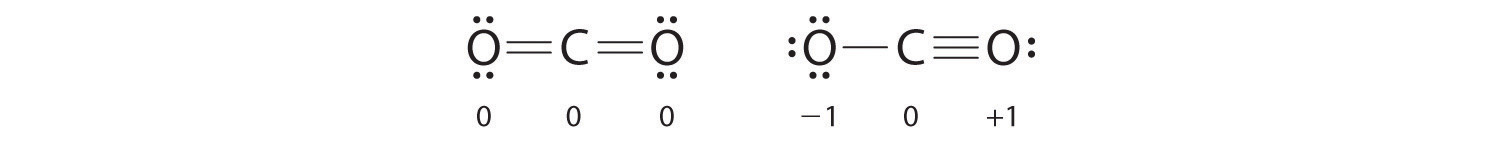
1 3 Lewis Structures Chemistry Libretexts
The concept of Lewis structures primarily relies.

. Each fluorine has 1 bond. The steric number the number of atoms bonded to the atom the number of lone pairs the atom has. So to determine these shapes around the central atom and the bond angles of these compounds we want to draw their LewiS structures.
In general an atom with all single bonds is an sp 3 hybridized. EG tetrahedral MG tetrahedral b. So starting with part A.
Iodine has 5 bonds and 1 lone electron pair. Answer 1 of 2. The best example is the alkanes.
Hydrogen will only have two electrons. Just take a solid object with high tensile strength and place it in environment with temperature equal to -27315C or 0k it will be arranged in a definite order as there is. And in this particular case who is going to be young people want to an oxygen and then.
Group 3A boron aluminum etc. EXCEPTIONS TO THE OCTET RULE. If all of the atoms usually form the same number of bonds.
So for this question to determine the shape around the central atom and the bond angles were going to draw the Lewis structure. Determining the Hybridization. The questions asked us to remain the more local structure for the following So far a be hell u n o.
The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. Count the bonds and lone electron pairs connected to the atom. EG - linear MG - bent c.
Help from article of Jack Brubaker. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The atom with the lowest ionization energy and the lowest electron affinity is the central atom.
If there are carbon atoms in an organic molecule carbons are most. Determine the electron geometry EG and molecular geometry MG of the underlined atom CH3CH3. How do you know if an atom is sp2 hybridized.
You can find the hybridization of an atom by finding its steric number. Arrange electrons around the atoms so that each atom has an octet. We have aged to S.
All the carbon atoms in an alkane are sp 3.

Nxnrug5nevaj0m
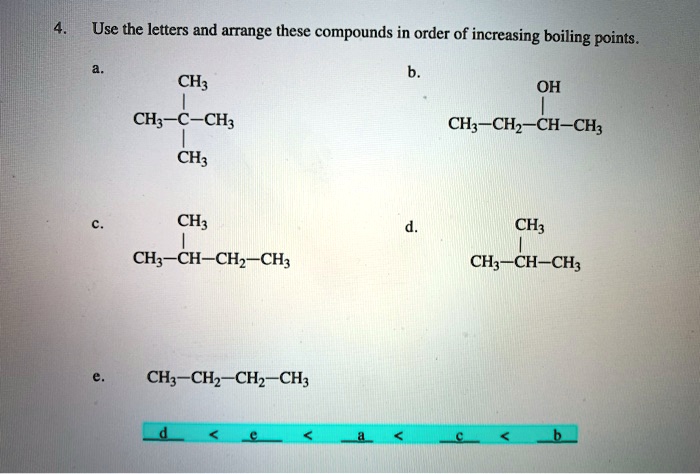
Browse Questions For Chemistry 101
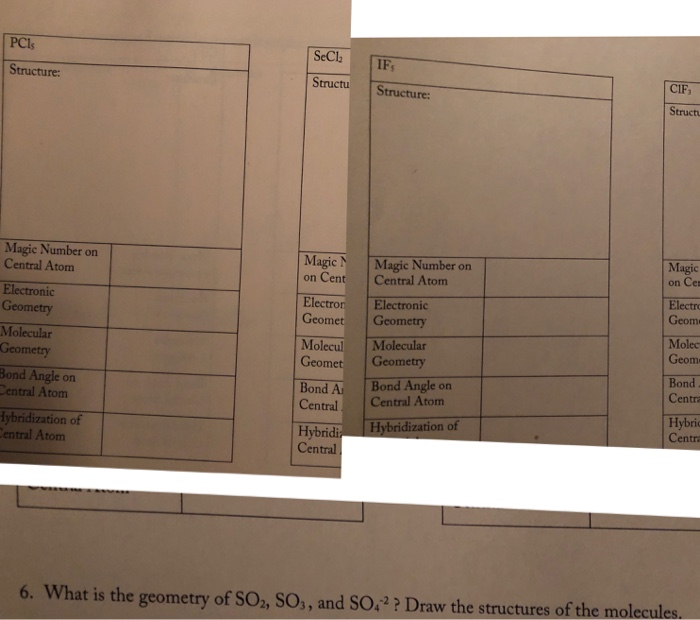
Solved Q5 Complete Tables Structure Magic On Central Chegg Com
Atoms Free Full Text Radiative Recombination And Photoionization Data For Tungsten Ions Electron Structure Of Ions In Plasmas

A Mass Spectrometry Based Framework To Define The Extent Of Disorder In Proteins Analytical Chemistry
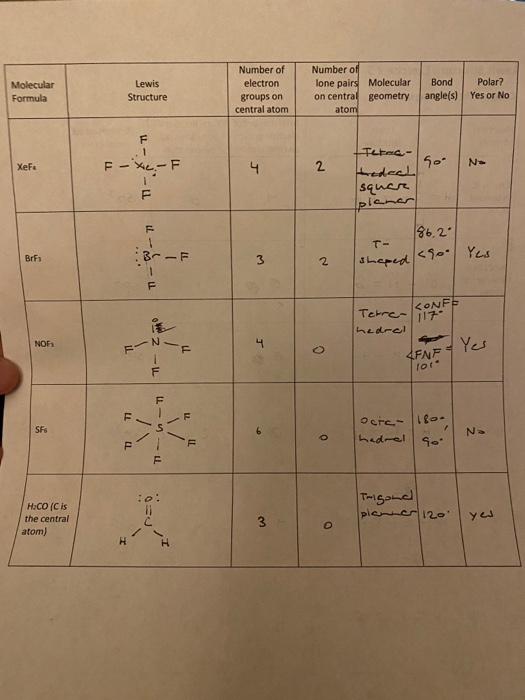
Solved Molecular Formula Lewis Structure Number Of Electron Chegg Com

Solved Compound Nh Valence Electrons Lewis Structure Chegg Com
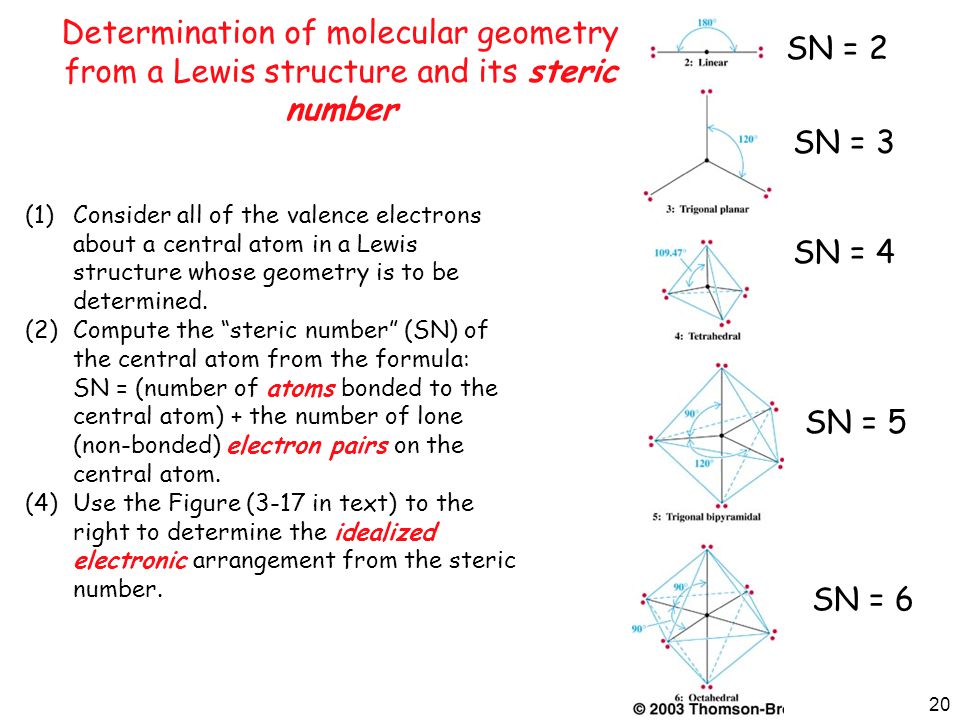
1 Covalent Bonding Sharing Of Electron Pairs By Atoms Ppt Video Online Download
Lewis Structure Calculator Online Solver With Free Steps
How Can We Determine The Central Atom In A Molecule While Making A Lewis Structure Or A Resonance Structure Quora

How To Determine Which Atom To Use As The Central Atom Sciencing

Chapter 5 Molecules With More Than 1 Central Atom Section 5 8 Youtube
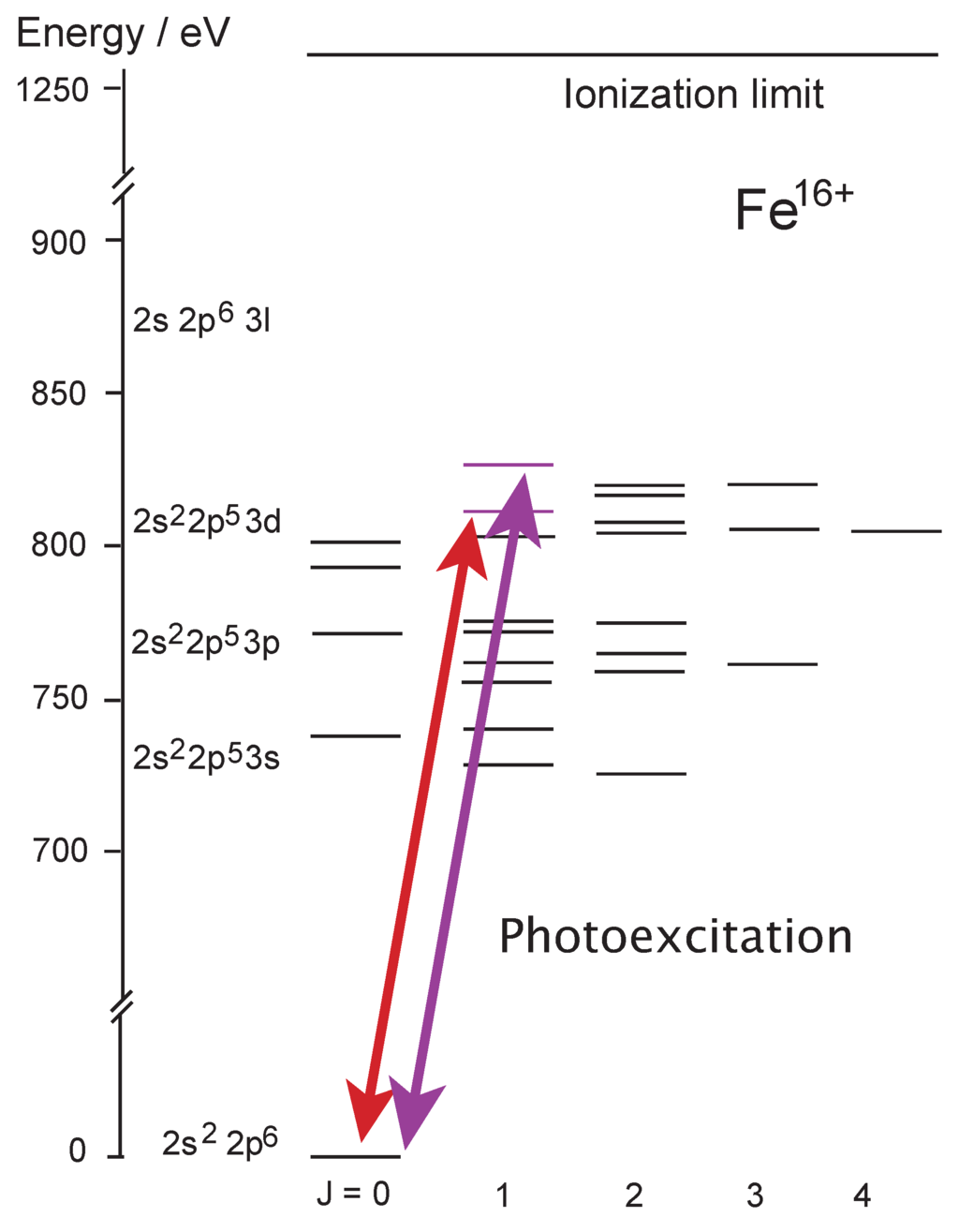
Atoms Free Full Text Critical Assessment Of Theoretical Calculations Of Atomic Structure And Transition Probabilities An Experimenter S View
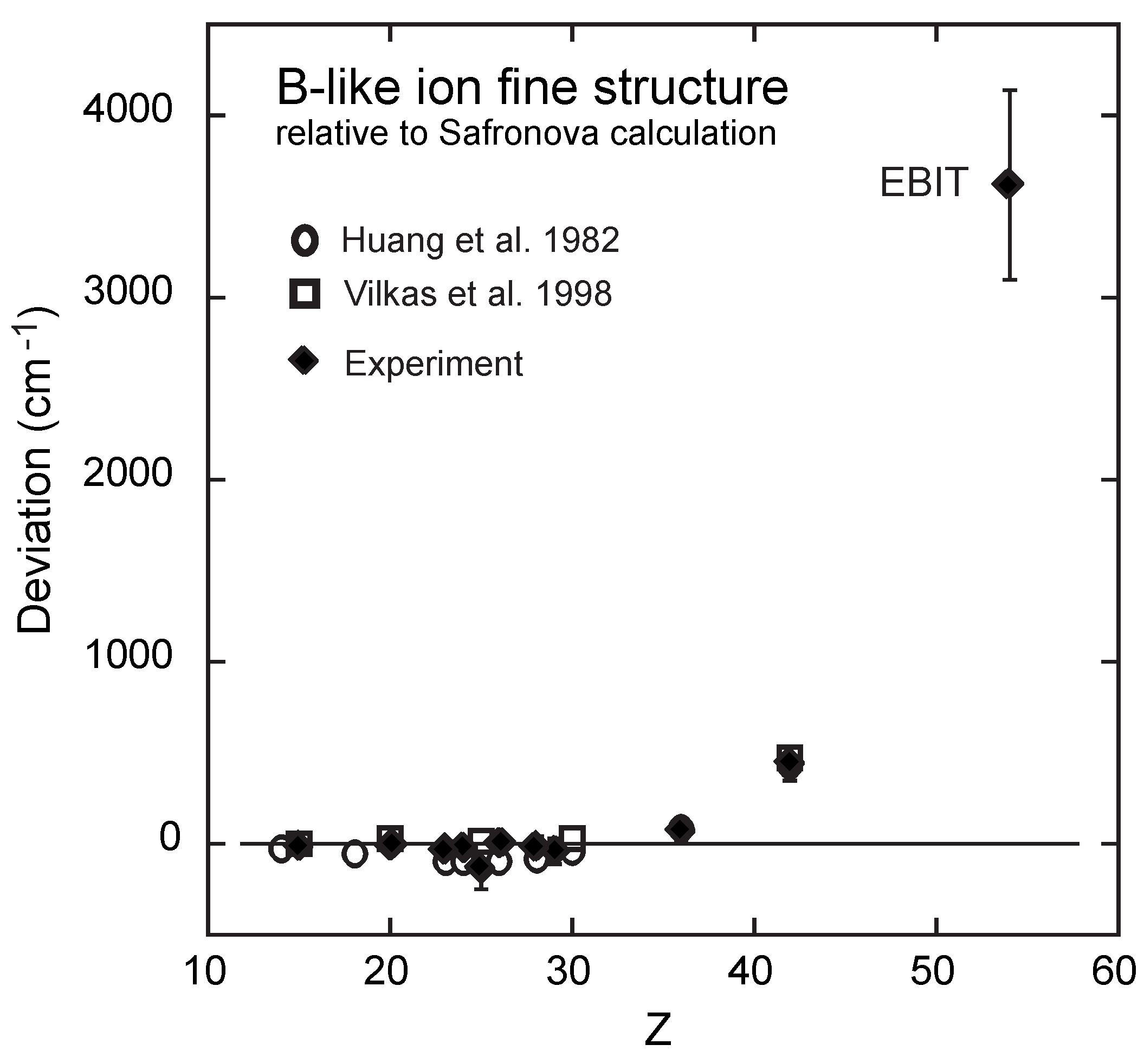
Atoms Free Full Text Critical Assessment Of Theoretical Calculations Of Atomic Structure And Transition Probabilities An Experimenter S View

Aleks Identifying A Molecule With One Central Atom From Its 3d Shape Youtube
The Atomic Mass Of An Element Is 27 If Valency Is 3 What Will Be The Vapour Density Of Chloride Quora

How To Identify Central Atom Trick For Identify Central Atom Concept Booster Series 04 Youtube